Abstract
Introduction: Representing 4% of central nervous system (CNS) tumors, primary CNS lymphoma (PCNSL) is a rare type of extranodal non-Hodgkin lymphoma (NHL) that involves the brain, leptomeninges, eyes, spinal cord or cerebrospinal fluid without evidence of systemic disease. High-dose methotrexate (HD-MTX)-based regimens that incorporate consolidation with reduced dose whole brain radiation therapy (WBRT) or autologous stem cell transplant have shown 2-year overall survival (OS) rates ranging from 69% to 81% (Morris, JCO, 2013; Omuro, Blood, 2015).
Although these data are encouraging, patients whose CNS deficits are too severe to receive treatment will die within the first months of diagnosis. HIV PCNSL has been associated with a poorer performance status and worse OS compared to non-HIV PCNSL. With the advent of combination antiretroviral therapy (ART), HIV PCNSL has become less common and by incorporating HD-MTX into HIV PCNSL treatment, outcomes have improved (Gupta, Neuro Oncol, 2017).
In this study, we report the real world survival of PCNSL at 3 large academic centers, including those who received HD-MTX versus those who did not. We also compare survival of HIV PCNSL relative to non-HIV PCNSL.
Methods: Adult patients (>18 years of age) with PCNSL at 3 medical centers (UNC Chapel Hill, Vanderbilt Medical Center, Emory University) were included in this analysis. Patients were diagnosed between January 2004 and July 2020. Only the diffuse large B-cell lymphoma (DLBCL) histology was included. Cases were excluded if there was any disease outside of the CNS or if they previously had systemic DLBCL. Demographic information was collected from the medical record, as well as disease-related information, HIV status, HD-MTX treatment (defined as >3g/m2) with or without other chemotherapeutic agents, imaging to determine progression, and survival data. Data were analyzed for the entire cohort as well as compared between HIV and non-HIV subjects. Demographic variables were summarized using appropriate statistics (frequencies, mean and standard deviation) and compared between HIV and non-HIV patients using Fisher's exact tests or Wilcoxon rank sum tests. Progression free survival (PFS) and OS were also compared between HIV and non-HIV patients using log-rank tests.
Results: We identified 158 cases of PCNSL. The median PFS for the entire cohort was 1.17y and the median OS was 3.24y. Patients who received HD-MTX had an improved PFS (1.69y vs 0.25y; p=0.0016) and an improved OS (4.01y vs 1.05y; p=0.00075) over those who did not receive HD-MTX.
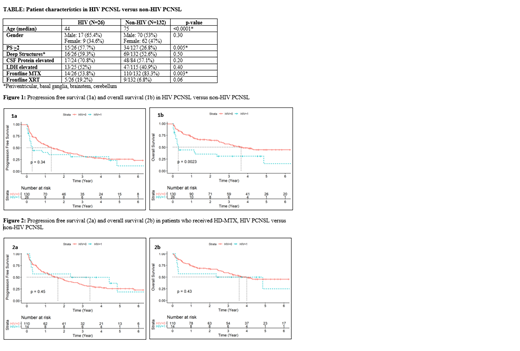
Twenty-six of the 158 cases were HIV positive. Table 1 reports clinical features in HIV versus non-HIV PCNSL patients. Patients with HIV PCNSL were significantly younger than those with non-HIV PCNSL (44y vs 75y), and had a worse performance status (PS>2: 57.7% vs 26.8%). Notably, patients with HIV were less likely to receive HD-MTX compared to patients without HIV (53.8% vs 83.3%). Receiving frontline WBRT trended towards significance in favor of the HIV PCNSL group. Other factors such as gender, deep structure involvement, elevated cerebrospinal fluid protein, and elevated lactose dehydrogenase (LDH) were not significantly different between groups.
When comparing HIV to non-HIV PCNSL, there was not a statistically significant difference in PFS (0.30y vs 1.34y; p=0.34), but there was a statistically significant difference in OS (0.30y vs 3.65y; p=0.0023) (Figure 1). When comparing those who received HD-MTX in the HIV group vs non-HIV group, there was not a statistically significant difference in PFS (3.39y vs 1.66y; p=0.45) or OS (3.6y vs 4.0y; p=0.43) (Figure 2).
Conclusions: Our analysis shows that real-world survival for PCNSL is modest compared to reported outcomes from clinical trials. Patients who are able to receive treatment with HD-MTX had a significantly improved outcome. HIV PCNSL has worse OS than non-HIV PCNSL, and the median PFS and OS were both several months in the HIV PCNSL group, suggesting an unmet need for both frontline and salvage therapies. The survival difference between HIV PCNSL and non-HIV PCNSL decreased when evaluating only those patients who received HD-MTX. Importantly, fewer patients received HD-MTX in the HIV PCNSL group; and these patients had worse PS. These real-world data may serve as the benchmark for survival outcomes in PCNSL and provide valuable information for designing future trials in patients with PCNSL.
Dittus: Genentech: Research Funding; AstraZeneca: Research Funding; Seattle Genetics: Research Funding; BeiGene: Other: Advisory Board. Grover: Kite: Other: Advisory Board; ADC: Other: Advisory Board; Novartis: Consultancy; Tessa: Consultancy; Genentech: Research Funding. Cohen: Janssen, Adicet, Astra Zeneca, Genentech, Aptitude Health, Cellectar, Kite/Gilead, Loxo, BeiGene, Adaptive: Consultancy; Genentech, BMS/Celgene, LAM, BioINvent, LOXO, Astra Zeneca, Novartis, M2Gen, Takeda: Research Funding. Park: G1 Therapeutics: Consultancy; Takeda: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Teva: Consultancy, Membership on an entity's Board of Directors or advisory committees; Morphosys: Membership on an entity's Board of Directors or advisory committees; Rafael Pharma: Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board; Seattle Genetics: Research Funding, Speakers Bureau; Gilead: Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal